We’re cleaning up what precision oncology leaves behind
Vision
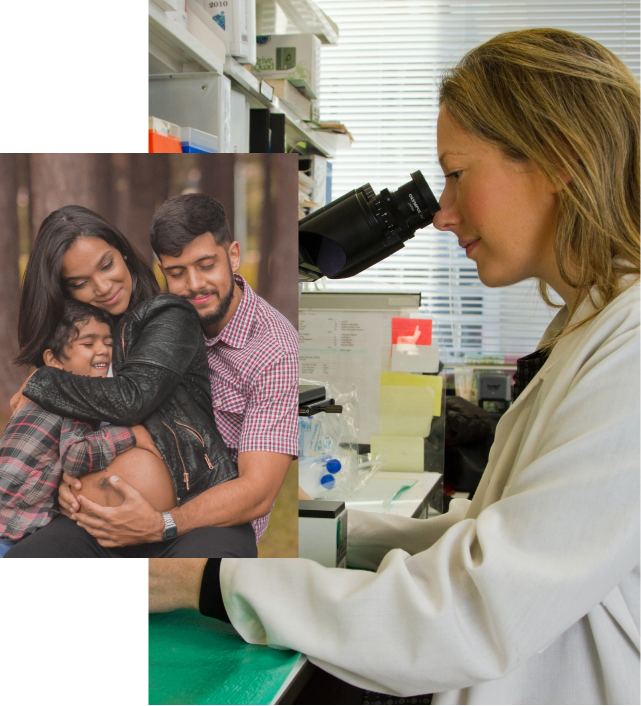
Concarlo’s vision is to create a world of possibility, hope and time by making cancer a treatable, manageable and survivable condition


Mission
Concarlo’s mission is to dramatically improve outcomes for patients with drug-resistant cancers by creating transformative therapies that control the p27 target.

Values
Be passionate
Communicate with openness, honesty, and transparency
Give trust and hold ourselves
accountable
Have a mentoring mindset
Derive joy from our work
Drive value
Meet Our Team
We've assembled an
experienced team of
purpose-driven,
world-class leaders in
science and business
Founder, CEO
Biography
Dr. Blain is an internationally known expert in cell cycle and cancer biology, and is one of the world’s experts on p27Kip1. She has been studying cell cycle regulation for more than 25 years as an NIH funded investigator and tenured Associate Professor at the SUNY Downstate Medical Center. She founded Concarlo, capitalizing on discoveries she made and patented. She was trained at Princeton, Columbia and the Memorial Sloan-Kettering Cancer Center.
Executive Chairman
Biography
Arthur Klausner is an experienced life science venture capitalist and company executive. He earned his M.B.A. from the Stanford University Graduate School of Business and his B.A. in Biology from Princeton University. He was previously CEO and a board member of Goldilocks Therapeutics, Gem Pharmaceuticals, and Jade Therapeutics. He earlier spent a total of 18 years as a life sciences venture capitalist at Domain Associates and Pappas Ventures. He currently serves on the Board of Directors of Monopar Therapeutics (Nasdaq: MNPR), and on the Life Sciences Investment Review Board for the New York University Innovation Venture Fund.
Chief Development Officer
Biography
Dr. Allamneni is a biopharma R&D expert with almost 20+ IND/IMPD submissions, 6 NDA/MAA approvals, and a track record of efficiently developing therapeutic candidates of various modalities. Forward-thinking leader in R&D strategy and tactical planning, and a highly regarded operational manager for key R&D functions. Impactful global regulatory interactions with FDA, MHRA, BfArM, ANSM, PMDA, Health Canada, TGA etc. during clinical development, partnership, and commercialization. Formerly with Turning Point, Jazz Pharmaceuticals and Genentech.
President
Biography
Mr. Mraz’s career has been focused in the financial markets for over 30 years since he joined JPMorgan in 1989. In 1999 he was a co-founding partner of the money management firm, Ospraie Management, where he is still an active partner today.In 2015 he teamed with Dr. Stacy Blain to found Concarlo and guides the team on all financial mattersIn 2017, he lead the development of the Ospraie AgScience team and platform, which currently invests in catalytic agricultural biotechnology.
Director, Chemistry, Manufacturing and Controls
Biography
Natalia specializes in liposome and lipid nanoparticle drug delivery systems. Prior to her current role, she held various positions at Evonik Vancouver laboratories, facilitating transition of customer projects through various stages of development, from proposal scoping to formulation development to early clinical manufacturing. Natalia has over 20 years of experience in lipid nanoparticle drug delivery field and has been involved in all aspects of liposome manufacturing, starting from early-stage formulation design, process development, scale-up and early clinical manufacturing. Prior to Evonik, she worked at a number of Vancouver based drug delivery companies (CDRD/adMare, Celator/Jazz, Inex), focusing on liposomal products. Natalia holds undergraduate degree in Chemistry from University of British Columbia.
Director of Research
Biography
Dr. Grace Chen received her Ph.D. in immunology at the University of Toronto, Canada. Following that, Grace completed two postdoctoral fellowships, published extensively, and have led numerous research projects. She has over 15 years of experience in the fields of cell biology, immunology, and hematology. Prior to her current role, she served as a research scientist at Concarlo where she spearheaded the optimization and preclinical development of Concarlo’s lead compound.
Research Scientist
Biography
Dr. Carolina Guido is a molecular biologist with a strong background in molecular and cellular biology. During her master’s thesis she obtained significant training in genetic engineering, as she investigated the use of homologous recombination as a tool to establish stable transfection in mammalian cells. In 2020, she completed her PhD at the National University of Córdoba, Argentina. Here she studied the participation of stem cells and cancer stem cells in endocrine tumors. She developed and published significant results as well as established mentoring skills as a professor with a focus in the Medicine career trajectory. She joined Dr. Stacy Blain’s laboratory at the State University of New York (SUNY Downstate) as a postdoctoral fellow focusing on pancreatic and breast cancer cell lines and cell cycle regulation through p27 regulation. She will be taking her passion and skills from this experience to enhance Concarlo’s research efforts.
Peptide Advisor
Biography
Dr. Bridon is an R&D executive with extensive North American and international experience of drug development from research to proof-of-concept clinical studies (Phase II) and regulatory submission in EU and US. 20+ years of experience with peptides therapeutics including drug protein bio-conjugate development across multiple therapeutic areas to treat cancer, anti-infective, metabolic and cardiovascular diseases. He previously held various management positions at Optivia (acquired by BioIVT), Ipsen, Abbott, Enobia (acquired by Alexion) and Neuronax and served on the scientific boards of Syntaxin (acquired by Ipsen) and Biosortia. He is currently CEO of Diaccurate, a Paris-based star-up.
Chemistry Advisor
Biography
Dr. DeVita is an experienced medicinal chemist and drug hunter in academia, biotech and large pharmaceutical environments. He has over 30 years of drug discovery and development experience including expertise managing multi-disciplinary teams to deliver on key program objectives for complex molecular targets. Dr. DeVita started his career at Merck Research Laboratories and rose to Director of Medicinal Chemistry, where he delivered many development candidates and led drug discovery and development teams. Dr. DeVita was also VP of Chemistry at Agios Pharmaceuticals focused on oncology drug discovery. Since 2013, he founded a consulting firm which has consulted for a wide variety of clients including biotechs building translational drug discovery programs. He is also Professor in the Dept. of Pharmacological Sciences and Director of Medicinal Chemistry in the Drug Discovery Institute of Icahn School of Medicine at Mt. Sinai since 2014.

Executive Assistant
Biography
Jen Imperial is an Executive Assistant at Concarlo Therapeutics supporting the Chief Scientific Officer and Chief Development Officer. She joined Concarlo Therapeutics in 2021. Prior to joining Concarlo Therapeutics, she held administrative, marketing, operations, and project management roles at various start up companies.
Meet Our Scientific
Advisors
Biography
Dr. Paz is an experienced executive in the biotech and pharma space, with demonstrated leadership and expertise in precision medecine, preclinical and clinical drug development and business development. She possesses profound, applicable scientific know-how of molecular and cellular biology, biochemistry, immunology, pharmacology, CMC, regulatory and clinical trial design. Dr. Paz is currently the Chief Development Officer at Nectin Therapeutics. Prior to that she held various leadership roles in biopharma companies, including ImClone Systems, Eli Lilly, Champions Oncology, Fortress Biotech and Stelexis Therapeutics. Keren holds a PhD from The Weizmann Institute of Science in Israel and was trained at The Rockefeller University and MSKCC under the mentoring of world-renowned scientists and Nobel laureates.
Biography
Dr. Schwartz is a board-certified medical oncologist, chief of Columbia University Medical Center’s Division of Hematology and Oncology and Deputy Director of the Herbert Irving Comprehensive Cancer Center. He is actively involved in translational and clinical research. His research studies have been supported by the National Cancer Institute, the Lustgarten Foundation for Pancreatic Cancer, the Department of Defense for Breast Cancer Research, the Byrne Foundation, and the Food and Drug Administration. He has served on the editorial boards of various scientific journals, including Associate Editor for the Journal of Clinical Oncology and Clinical Cancer Research. He is also actively involved in cooperative group activities and serves on the Board of Directors and Executive Committee of the Alliance for Clinical Trials in Oncology and is co-Chair of the Experimental Therapeutics Committee for Rare Cancers.
Biography
Andrew koff is the Head of the Laboratory of Cell Cycle Regulation Sloan-Kettering Institute. He also serves as a professor at the Molecular Biology Program of Weill Cornell Medical College. His laboratory is interested in the mechanisms that control the decision of mammalian cells to either commit to the mitotic proliferative cycle or exit the cell cycle and differentiate, and the effect that mis-regulation of this decision has on development of a tissue and on tumor growth
Biography
Dr. Shapiro received his PhD in 1987 and his MD in 1988 from Cornell University, followed by postgraduate training in internal medicine at Beth Israel Hospital, Boston, where he served as chief medical resident. He completed a fellowship in medical oncology at Dana-Farber Cancer Institute, during which he investigated the role of cell-cycle-related proteins in lung cancer. Dr. Shapiro’s practice is focused in the Center for Cancer Therapeutic Innovation, where he develops and leads early phase clinical trials. As Senior Vice President, Developmental Therapeutics, he also works integrally with multiple disease groups within the Institute.
Biography
Neal Rosen MD, PhD is a member of the Program in Molecular Pharmacology and the Department of Medicine at Memorial Sloan-Kettering Cancer Center where he also is the incumbent of the Enid Haupt Chair in Medical Oncology. He has pioneered the ideas that oncogene-induced negative feedback plays important roles in determining mechanisms of tumorigenesis and progression and that its relief causes adaptive resistance to targeted therapies. He has also played important roles in developing therapeutic strategies for inhibition of RAS/RAF/MEK or PI3K/mTOR signaling.
Biography
Mary Mader is a medicinal chemist whose career has spanned pharmaceutical, biotech and not-for-profit drug discovery. She contributed to the development of multiple clinical candidates for oncology indications at Eli Lilly and Company and Relay Therapeutics, focused on kinase and epigenetic targets. She served as chair of the medicinal chemistry Gordon Research Conference (2010), the inaugural medicinal chemistry Gordon Research Seminar (2013), the Chemistry in Cancer Research working group of the AACR (2023-2024), and the Expert Scientific Advisory Committee of the Medicines for Malaria Venture (2023-2028) and is on the editorial board of Molecular Cancer Therapeutics. She received her PhD from the University of Notre Dame in organic chemistry and completed an NIH post-doctoral fellowship at the University of California, Berkeley.
Biography
Dr. Simon Eastman received a BSc Honours degree in Biochemistry from Carleton University in 1986. His post-graduate studies were performed in the laboratory of Dr. Pieter Cullis at the University of British Columbia where he studied lipid asymmetry in model membranes and received his Ph.D. in 1991. His post-doctoral studies were undertaken in the laboratory of Dr. Ben de Kruijff at the University of Utrecht in the Netherlands.
From 1992 to 2002, Dr. Eastman worked as a research scientist at Genzyme Corporation in Framingham Massachusetts. There, he was involved in the development of non-viral gene therapy formulations. His work focused on developing an aerosolized formulation of cationic lipid:plasmid DNA to transfect the lungs of Cystic Fibrosis patients with a plasmid encoding for the cystic fibrosis transmembrane regulator (CFTR). This work led to the first clinical trial with an aerosolized non-viral gene therapy vector to the lungs of CF patients.
In 2002 Dr. Eastman moved to Northern Lipids Inc (now Evonik) where he was the Director of Formulation Development. NLI was a small CRO/CMO focused on the development of liposomal formulations of small molecules and macromolecules (proteins and nucleic acid based therapies). He directed scientific studies from early-stage programs through to scale up and technology transfer of processes to GMP facilities, both internally at NLI as well as to external CMO’s. At NLI Dr. Eastman participated in the design of facilities and custom equipment to allow for the safe manufacture of formulations containing potent API’s and for developing novel processes for the manufacture of numerous formulations (e,g. mixing equipment for the production of genetic material with lipids to form lipid nanoparticles).
From December of 2014 until July 2023, Dr. Eastman was Vice President of Global Manufacturing and Technical Operations at ProNai Therapeutics (subsequently Sierra Oncology). He was responsible for overseeing the manufacturing of Drug Substance and Drug Product, development of a secure supply chain, and overseeing analytical programs including development, qualification and validation. Sierra Oncology was acquired by GSK in July of 2022, following the filing of an NDA for Momelotinib which was approved in June of 2023. Dr. Eastman was retained by GSK until July 2023 to support tech transfer and participate in regulatory fillings (MAA and JP filings).
Meet Our Business
Advisors
Biography
Mike Miller has over 40+ years of pharmaceutical and biotech industry experience. He has built/led large teams and all facets of commercialization, including General Management, Marketing, Sales Management, Market Access, Analytics, Market Research and Launch Planning. Therapeutic area experience includes Hematology Oncology, Solid Tumors (breast, lung, prostate, pancreatic), Neurology, Sleep Medicine, Reproductive Endocrinology, OBG, Pain, Urology, Cardiology, Immunology, Dermatology, and Metabolic Disease. Mike has public and non-profit BOD experience, as well as consulting experience with private and public companies in early and late stage drug development on commercial/market assessment and market access. Mike currently serves on the board of directors of BioXcel Therapeutics and PUMA Biotechnology.
Biography
Mike Harrington is a corporate executive and board member with 25 years of experience building global organizations and promoting corporate priorities in the heavily regulated life sciences industry. He has a history of increasing value across Eli Lilly’s worldwide operations through long-range vision, operational excellence and acquisitions. He provides a multidimensional perspective on corporate governance, strategic planning, enterprise risk management, and information security in regulated environments. He is also a current board member at Elanco Animal Health.
Biography
Jay Campbell is a life sciences and finance executive with over 15 years of experience in the financial services industry, including 13 years focused on the life sciences as an investment banker working with private/public companies on strategic/M&A and financing transactions. He also has eight years of biotechnology industry experience working at private/public companies with core responsibilities of alliance management, business/corporate development, capital raising, and investor relations. Jay is currently Managing Director of the Clinical Accelerator and Venture fund at the Cancer Research Institute where he oversees the organization’s clinical trials and venture investing. He has successfully worked on 36 strategic and financing transactions and investments representing over $13.4 billion. Jay has worked at Immutep Limited, Kolltan Pharmaceuticals, Maxim Group, ABN AMRO/Royal Bank of Scotland, BIO-IB, ABN AMRO Rothschild, and Schroder & Company.
Biography
Alicia Chung is the Sr Vice President of Strategy and Business Development at Bionaut Labs, leading the company’s corporate strategy and business partnerships. Previously, she served as Head of Corporate Development at Amphivena Therapeutics, Inc, a clinical stage immunotherapy company, where she was responsible for the company’s fundraising, partnership transactions, and company operations. Prior to that, Alicia led Strategy and Business Development at Halozyme Therapeutics where she executed out-licensing of Halozyme’s drug delivery technology platform and early stage oncology assets. Alicia began her career at Roche-Genentech, where she held various positions of increasing responsibility in R&D, commercial and business development and licensing.
“If your actions inspire others to dream more, learn more, do more, and become more, you are a leader.”
—John Quincy Adams
Our Partners





Our Investors